BACKGROUND:
Classic Hodgkin lymphoma (cHL) Nodular Sclerosis (NS) is the most common subtype of cHL and has a good prognosis. However, an uncommon variant of cHL NS, the syncytial variant (cHL NS-SV), represents an entity with a worse clinical outcome with conventional frontline cytotoxic therapy. Nevertheless, in the pivotal phase III study ECHELON-1, Brentuximab Vedotin (BV), doxorubicin, vinblastine, and dacarbazine (BV+AVD) while demonstrating a superior efficacy, unfortunately more febrile neutropenia (FN) and peripheral neuropathy (PN) were observed compared to bleomycin+AVD (ABVD) as frontline treatment of advanced-stage classical Hodgkin's lymphoma (cHL) (Straus et al Blood 2020). It is unknown whether BV combined with chemotherapy would improve the inferior prognosis of cHL NS-SV.
METHODS:
After obtaining institutional research approval, we retrospectively reviewed the characteristics and outcomes of adult cHL NS-SV patients, treated per physician preference with frontline BV combined with cytotoxic therapy, either as standard of care (SOC) or in a clinical trial (NCT01712490, NCT03646123 and NCT01060904) between 7/2010 and 7/2020. cHL NS-SV diagnosis was based on morphology and Immunophenotype. Bulky disease was defined as mass>10cm or mediastinal mass >1/3 of thoracic diameter at T5-T6. Lymph node regions were defined by the German Hodgkin Study Group (GHSG). Treatment consisted of 0.9 or 1.2 mg/kg of BV and standard dose ABVD or AVD or D, intravenously on days 1 and 15 of each 28-day cycle for up to 6 cycles. All patients received support with pegfilgrastim. Response assessment was based on positron emission tomography/computerized tomography (PET/CT). Follow-up time was calculated from the date of pre-treatment baseline lab measurement to relapse date or death date, whichever happened first. Patients with no event were censored at the last follow-up date.
RESULTS:
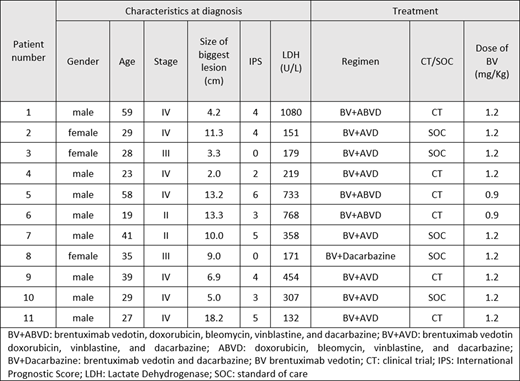
Among the 11 patients included in the analysis, the median age was 29 (range 19-59 years), 27.2% were female, and 90.9% were Caucasian. All patients had Eastern Cooperative Oncology Group (ECOG) 0-1, 72.7% of patients had B-symptoms and 81.9% had stage III or IV disease. The median size of the largest lesion was 9.0 cm, 45.5% of patients had bulky disease, 63.6% had extranodal disease, 72.7% had >3 GHSG involved nodal sites and median International Prognostic Score was 4.
Overall, all patients received 6 cycles of therapy, 63.6% received BV+AVD, 27.3% received BV+ABVD and 1 patient received SOC BV with dacarbazine because of previous anthracycline exposure.
The interim PET/CT showed response in all patients with Deauville score (DS) of 2-3 in 45.5% and 4 in 54.4%. The end-of-therapy PET/CT showed DS of 1-3 in 90.1% and 4 in 8.9%. The latter patient had the most voluminous bulk of this study. To date, no patient received additional systemic therapy or consolidative radiotherapy after completion induction therapy. With a median follow-up time of 14 months (range 5-82 months), no patient had relapse and all were still alive (Progression-Free Survival and Overall Survival of 100%).
CONCLUSIONS:
BV combined with cytotoxic therapy such as BV+AVD is a highly effective treatment strategy for patients with cHL NS-SV. Further studies are required to evaluate whether frontline therapy of BV+AVD could improve the outcome of the higher-risk subgroup cHL NS-SV.
Lee:Oncternal Therapeutics:Research Funding;Guidepoint Blogal:Consultancy;Aptitude Health:Speakers Bureau;Takeda:Research Funding;Seattle Genetics:Research Funding;Celgene:Research Funding;Bristol-Myers Squibb:Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal